“Is progression-free survival time predictive of overall survival?
Analysis of studies of targeted therapies in oncology”
by G. Delépine, N. Delépine, S. Alkhallaf
presented in Bobigny congress 2017
Introduction
The duration of progression-free survival (PFS) is the first criterion of judgment of more than 90% of the trials of the current innovative therapies at the time of the MA application. This PFS is supposed to be predictive of overall survival and therefore of future usefulness for patients. This work aims to verify it as well as its predictive value of clinical utility for patients.
Method
A computerized search with key words Avastin, Herceptin, Erbitux, erlotinib, sorafenib, gefitinib, crizotinib, afatinib, temsirolimus, pazopanib, sunitinib, axitinib, in bronchopulmonary squamous cell carcinoma, kidney, colon, breast and ENT was realized. 110 trials were then examined to select those where SPP and long-term outcomes were published detailing overall survival and toxicity (51 studies). The gains in progression-free survival durations reported in the pivotal study were then compared to those of overall survival.
Results
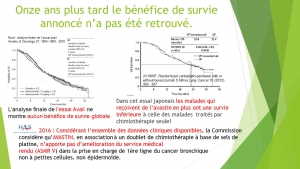
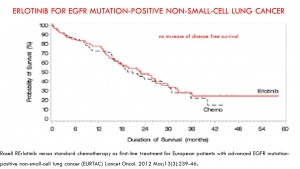
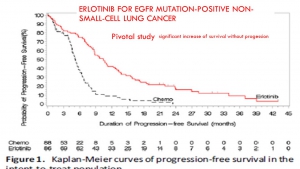
The progression-free survival gains reported by the pivotal studies of targeted therapies are not correlated with a possible overall survival benefit or with a potential gain in quality of life. This result confirms many macroanalyses including those of Petrelli and the Cochrane Institute (for TKI and lung cancers), and the Cochrane Institute (for Avastin and breast cancer).
Conclusions
In trials of targeted therapies in oncology, the duration of progression-free survival does not prejudge either the overall survival time or a favourable benefit / risk balance. Favouring this criterion to award the AMM results in exposing patients to significant risks and most often without any real benefit. It should therefore no longer be accepted as the main crite